Theoretical basis of organic reactions
Theoretical basis of organic reactions: Overview
This topic covers concepts such as organic reactions and mechanisms, organic reactions, bond cleavage, homolytic bond cleavage, and conditions for homolytic bond cleavage.
Important Questions on Theoretical basis of organic reactions
Write correct order of stability of following carbocations :
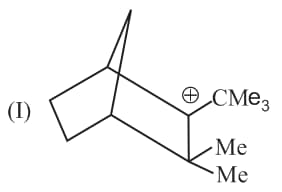
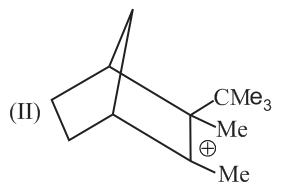
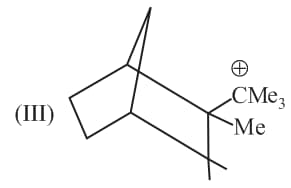
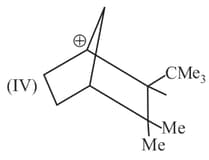
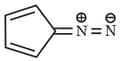
The most stable canonical structure of this molecule is:
In which of the following molecules / ions resonance structures are equivalent:
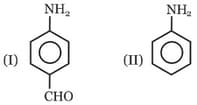
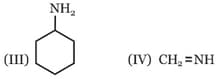
Among these compounds, the correct order of C–N bond length is :
Rank the following free radicals in order of decreasing stability:
In which of the following pairs, is the first species is more stable than second?
In which of the following molecules π-electron density in ring is maximum ?
In which of the following molecules π-electron density in ring is minimum ?
Which of the following compounds has maximum electron density in ring ?
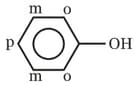
In phenol, π - electron - density is maximum on
The order of stability of these canonical forms is:
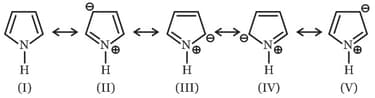
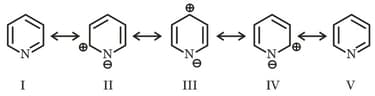
Among these canonical structures of pyridine, the correct order of stability is
The most stable resonating structure of following compound is
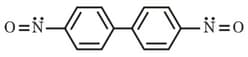
Among these three canonical structures (through more are possible) what would be their relative contribution in the hybrid?
Among these canonical structures which one is least stable ?
In which of the following molecules - group is not coplanar with phenyl ring?
In which of the following molecules resonance takes place through out the entire system
The increasing order of basic strengths in their aqueous solutions is:
The correct stability order for the following species is
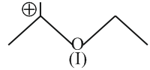
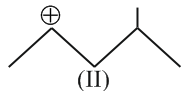
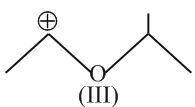
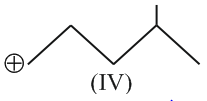
Hyperconjugation involves overlap of the following orbitals
